STRUCTURE OF ATOMS LECTURE 1 | Rutherford's Atomic Model
CHAPTER 2
STRUCTURE OF ATOMS
LECTURE 1
The structure of atom refers to how the sub-atomic particles
are arranged. Atoms are so small that they can only be visualized with a
scanning tunneling microscope.
Atom
The word
‘atom’ was first introduced by Greek philosopher Democritus. He gave the
concept that matter is made up of very small indivisible particles called
atoms. The word atom means indivisible, uncuttable (a-not + tomos-cutting).
The sub-atomic particles in an atom are protons (positively charged), electrons
(negatively charged) and neutrons (neutral – no charge).
Rutherford’s Atomic Model
In 1911,
Rutherford performed an experiment in order to know the arrangement of
electrons and protons in atoms. Rutherford's atomic model shows the existence
of nucleus in the atom, nature of charge on the nucleus and the magnitude of
charge on the nucleus.
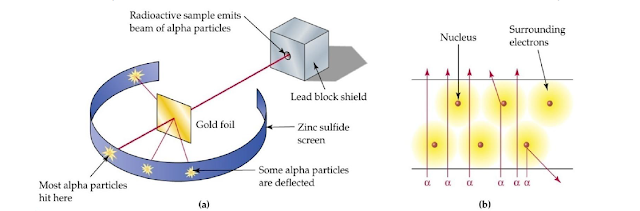
Rutherford’s Experiment
Apparatus for Experiment
- Very thin gold foil of about 0.0004cm thickness.
- α particles obtained from the disintegration of polonium.
- Zinc sulphide screen (ZnS).
Experiment
 |
| Figure 1 Rutherford's Experiment |
Properties of α-particles
α-particles are helium
nuclei that are doubly positively charged (He++).
Observations
Most of the α-particles are passed straight through the
foil. Only few particles were slightly deflected. But one in million (1/100000)
was deflected through an angle greater than 90ο from their straight
paths. Rutherford performed a series of experiments using thin foils of
other elements. He observed similar results from these experiments.
Conclusions
From his experiments, Rutherford proposed a new model for an
atom. He proposed a planetary model (similar to solar system) for an atom. An
atom is a neutral particle. The mass of an atom is concentrated in a
very small dense positively charged region. He named this region as a nucleus. The electrons are revolving
around the nucleus in circles. These circles are called orbits. The centripetal force due to the revolution of electrons
balances the electrostatic force of attraction between the nucleus and the electrons.
Rutherford drew the following conclusions:-
- Since majority of the α-particles passed through the foil un-deflected, most of the space occupied by an atom must be empty.
- The deflection of a few α-particles through angles greater than 90ᵒ shows that these particles are deflected by electrostatic repulsion between the positively charged α-particles and the positively charged part of atom.
- Massive α-particles are not deflected by electrons (See Figure 1 part b).
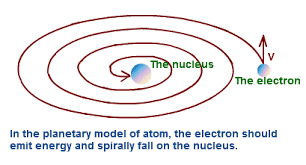
Defects in Rutherford’s Atomic Model
Rutherford’s model of an atom resembles our solar system. It
has following defects:-
- According to classical physics, electron being charged particle will emit energy continuously while revolving around the nucleus. Thus the orbit of the revolving electron becomes smaller and smaller until it would fall into the nucleus. This would collapse the atomic structure (see Figure 2).
- If revolving electron emits energy continuously it should form a continuous spectrum for an atom but actually a line spectrum is obtained.
 |
| Figure 2 Defect in Rutherford's Experiment |
 |
Figure 3 Difference between continuous spectrum and line
spectrum
|
Fundamentals of Chemistry Lecture 1
Fundamentals of Chemistry Lecture 2
Visit our Facebook page click here
Join our Facebook Group Shining Star Virtual Academy

Comments
Post a Comment